Example: example_4_rsmean
Modeling neuron-glia interactions with the Brian 2 simulator Marcel Stimberg, Dan F. M. Goodman, Romain Brette, Maurizio De Pittà bioRxiv 198366; doi: https://doi.org/10.1101/198366
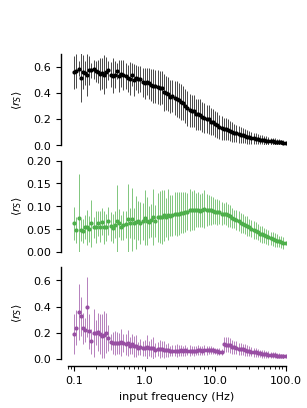
Figure 4C: Closed-loop gliotransmission.
I/O curves in terms average per-spike release vs. rate of stimulation for three synapses: one without gliotransmission, and the other two with open- and close-loop gliotransmssion.
from brian2 import *
import plot_utils as pu
set_device('cpp_standalone', directory=None) # Use fast "C++ standalone mode"
seed(1929) # to get identical figures for repeated runs
################################################################################
# Model parameters
################################################################################
### General parameters
N_synapses = 100
N_astro = 2
transient = 15*second
duration = transient + 180*second # Total simulation time
sim_dt = 1*ms # Integrator/sampling step
### Neuron parameters
# ### Synapse parameters
### Synapse parameters
rho_c = 0.005 # Synaptic vesicle-to-extracellular space volume ratio
Y_T = 500*mmolar # Total vesicular neurotransmitter concentration
Omega_c = 40/second # Neurotransmitter clearance rate
U_0__star = 0.6 # Resting synaptic release probability
Omega_f = 3.33/second # Synaptic facilitation rate
Omega_d = 2.0/second # Synaptic depression rate
# --- Presynaptic receptors
O_G = 1.5/umolar/second # Agonist binding (activating) rate
Omega_G = 0.5/(60*second) # Agonist release (deactivating) rate
### Astrocyte parameters
# --- Calcium fluxes
O_P = 0.9*umolar/second # Maximal Ca^2+ uptake rate by SERCAs
K_P = 0.05 * umolar # Ca2+ affinity of SERCAs
C_T = 2*umolar # Total cell free Ca^2+ content
rho_A = 0.18 # ER-to-cytoplasm volume ratio
Omega_C = 6/second # Maximal rate of Ca^2+ release by IP_3Rs
Omega_L = 0.1/second # Maximal rate of Ca^2+ leak from the ER
# --- IP_3R kinectics
d_1 = 0.13*umolar # IP_3 binding affinity
d_2 = 1.05*umolar # Ca^2+ inactivation dissociation constant
O_2 = 0.2/umolar/second # IP_3R binding rate for Ca^2+ inhibition
d_3 = 0.9434*umolar # IP_3 dissociation constant
d_5 = 0.08*umolar # Ca^2+ activation dissociation constant
# --- IP_3 production
# --- Agonist-dependent IP_3 production
O_beta = 3.2*umolar/second # Maximal rate of IP_3 production by PLCbeta
O_N = 0.3/umolar/second # Agonist binding rate
Omega_N = 0.5/second # Maximal inactivation rate
K_KC = 0.5*umolar # Ca^2+ affinity of PKC
zeta = 10 # Maximal reduction of receptor affinity by PKC
# --- Endogenous IP3 production
O_delta = 0.6*umolar/second # Maximal rate of IP_3 production by PLCdelta
kappa_delta = 1.5* umolar # Inhibition constant of PLC_delta by IP_3
K_delta = 0.1*umolar # Ca^2+ affinity of PLCdelta
# --- IP_3 degradation
Omega_5P = 0.05/second # Maximal rate of IP_3 degradation by IP-5P
K_D = 0.7*umolar # Ca^2+ affinity of IP3-3K
K_3K = 1.0*umolar # IP_3 affinity of IP_3-3K
O_3K = 4.5*umolar/second # Maximal rate of IP_3 degradation by IP_3-3K
# --- IP_3 diffusion
F_ex = 2.0*umolar/second # Maximal exogenous IP3 flow
I_Theta = 0.3*umolar # Threshold gradient for IP_3 diffusion
omega_I = 0.05*umolar # Scaling factor of diffusion
# --- Gliotransmitter release and time course
C_Theta = 0.5*umolar # Ca^2+ threshold for exocytosis
Omega_A = 0.6/second # Gliotransmitter recycling rate
U_A = 0.6 # Gliotransmitter release probability
G_T = 200*mmolar # Total vesicular gliotransmitter concentration
rho_e = 6.5e-4 # Astrocytic vesicle-to-extracellular volume ratio
Omega_e = 60/second # Gliotransmitter clearance rate
alpha = 0.0 # Gliotransmission nature
################################################################################
# Model definition
################################################################################
defaultclock.dt = sim_dt # Set the integration time
f_vals = np.logspace(-1, 2, N_synapses)*Hz
source_neurons = PoissonGroup(N_synapses, rates=f_vals)
target_neurons = NeuronGroup(N_synapses, '')
### Synapses
# Note that the synapse does not actually have any effect on the post-synaptic
# target
# Also note that for easier plotting we do not use the "event-driven" flag here,
# even though the value of u_S and x_S only needs to be updated on the arrival
# of a spike
synapses_eqs = '''
# Neurotransmitter
dY_S/dt = -Omega_c * Y_S : mmolar (clock-driven)
# Fraction of activated presynaptic receptors
dGamma_S/dt = O_G * G_A * (1 - Gamma_S) - Omega_G * Gamma_S : 1 (clock-driven)
# Usage of releasable neurotransmitter per single action potential:
du_S/dt = -Omega_f * u_S : 1 (event-driven)
# Fraction of synaptic neurotransmitter resources available for release:
dx_S/dt = Omega_d *(1 - x_S) : 1 (event-driven)
r_S : 1 # released synaptic neurotransmitter resources
G_A : mmolar # gliotransmitter concentration in the extracellular space
'''
synapses_action = '''
U_0 = (1 - Gamma_S) * U_0__star + alpha * Gamma_S
u_S += U_0 * (1 - u_S)
r_S = u_S * x_S
x_S -= r_S
Y_S += rho_c * Y_T * r_S
'''
synapses = Synapses(source_neurons, target_neurons,
model=synapses_eqs, on_pre=synapses_action,
method='exact')
# We create three synapses per connection: only the first two are modulated by
# the astrocyte however. Note that we could also create three synapses per
# connection with a single connect call by using connect(j='i', n=3), but this
# would create synapses arranged differently (synapses connection pairs
# (0, 0), (0, 0), (0, 0), (1, 1), (1, 1), (1, 1), ..., instead of
# connections (0, 0), (1, 1), ..., (0, 0), (1, 1), ..., (0, 0), (1, 1), ...)
# making the later connection descriptions more complicated.
synapses.connect(j='i') # closed-loop modulation
synapses.connect(j='i') # open modulation
synapses.connect(j='i') # no modulation
synapses.x_S = 1.0
### Astrocytes
# The astrocyte emits gliotransmitter when its Ca^2+ concentration crosses
# a threshold
astro_eqs = '''
# Fraction of activated astrocyte receptors:
dGamma_A/dt = O_N * Y_S * (1 - Gamma_A) -
Omega_N*(1 + zeta * C/(C + K_KC)) * Gamma_A : 1
# IP_3 dynamics:
dI/dt = J_beta + J_delta - J_3K - J_5P + J_ex : mmolar
J_beta = O_beta * Gamma_A : mmolar/second
J_delta = O_delta/(1 + I/kappa_delta) *
C**2/(C**2 + K_delta**2) : mmolar/second
J_3K = O_3K * C**4/(C**4 + K_D**4) * I/(I + K_3K) : mmolar/second
J_5P = Omega_5P*I : mmolar/second
delta_I_bias = I - I_bias : mmolar
J_ex = -F_ex/2*(1 + tanh((abs(delta_I_bias) - I_Theta)/omega_I)) *
sign(delta_I_bias) : mmolar/second
I_bias : mmolar (constant)
# Ca^2+-induced Ca^2+ release:
dC/dt = (Omega_C * m_inf**3 * h**3 + Omega_L) * (C_T - (1 + rho_A)*C) -
O_P * C**2/(C**2 + K_P**2) : mmolar
dh/dt = (h_inf - h)/tau_h : 1 # IP3R de-inactivation probability
m_inf = I/(I + d_1) * C/(C + d_5) : 1
h_inf = Q_2/(Q_2 + C) : 1
tau_h = 1/(O_2 * (Q_2 + C)) : second
Q_2 = d_2 * (I + d_1)/(I + d_3) : mmolar
# Fraction of gliotransmitter resources available for release
dx_A/dt = Omega_A * (1 - x_A) : 1
# gliotransmitter concentration in the extracellular space
dG_A/dt = -Omega_e*G_A : mmolar
# Neurotransmitter concentration in the extracellular space
Y_S : mmolar
'''
glio_release = '''
G_A += rho_e * G_T * U_A * x_A
x_A -= U_A * x_A
'''
astrocyte = NeuronGroup(N_astro*N_synapses, astro_eqs,
# The following formulation makes sure that a "spike" is
# only triggered at the first threshold crossing
threshold='C>C_Theta',
refractory='C>C_Theta',
# The gliotransmitter release happens when the threshold
# is crossed, in Brian terms it can therefore be
# considered a "reset"
reset=glio_release,
method='rk4')
astrocyte.h = 0.9
astrocyte.x_A = 1.0
# Only the second group of N_synapses astrocytes are activated by external stimulation
astrocyte.I_bias = (np.r_[np.zeros(N_synapses), np.ones(N_synapses)])*1.0*umolar
## Connections
ecs_syn_to_astro = Synapses(synapses, astrocyte,
'Y_S_post = Y_S_pre : mmolar (summed)')
# Connect the first N_synapses synapses--astrocyte pairs
ecs_syn_to_astro.connect(j='i if i < N_synapses')
ecs_astro_to_syn = Synapses(astrocyte, synapses,
'G_A_post = G_A_pre : mmolar (summed)')
# Connect the first N_synapses astrocytes--pairs
# (closed-loop configuration)
ecs_astro_to_syn.connect(j='i if i < N_synapses')
# Connect the second N_synapses astrocyte--synapses pairs
# (open-loop configuration)
ecs_astro_to_syn.connect(j='i if i >= N_synapses and i < 2*N_synapses')
################################################################################
# Monitors
################################################################################
syn_mon = StateMonitor(synapses, 'r_S',
record=np.arange(N_synapses*(N_astro+1)))
################################################################################
# Simulation run
################################################################################
run(duration, report='text')
################################################################################
# Analysis and plotting
################################################################################
plt.style.use('figures.mplstyle')
fig, ax = plt.subplots(nrows=4, ncols=1, figsize=(3.07, 3.07*1.33), sharex=False,
gridspec_kw={'height_ratios': [1, 3, 3, 3],
'top': 0.98, 'bottom': 0.12,
'left': 0.22, 'right': 0.93})
## Turn off one axis to display accordingly to the other figure in example_4_synrel.py
ax[0].axis('off')
ax[1].errorbar(f_vals/Hz, np.mean(syn_mon.r_S[2*N_synapses:], axis=1),
np.std(syn_mon.r_S[2*N_synapses:], axis=1),
fmt='o', color='black', lw=0.5)
ax[1].set(xlim=(0.08, 100), xscale='log',
ylim=(0., 0.7),
ylabel=r'$\langle r_S \rangle$')
pu.adjust_spines(ax[1], ['left'])
ax[2].errorbar(f_vals/Hz, np.mean(syn_mon.r_S[N_synapses:2*N_synapses], axis=1),
np.std(syn_mon.r_S[N_synapses:2*N_synapses], axis=1),
fmt='o', color='C2', lw=0.5)
ax[2].set(xlim=(0.08, 100), xscale='log',
ylim=(0., 0.2), ylabel=r'$\langle r_S \rangle$')
pu.adjust_spines(ax[2], ['left'])
ax[3].errorbar(f_vals/Hz, np.mean(syn_mon.r_S[:N_synapses], axis=1),
np.std(syn_mon.r_S[:N_synapses], axis=1),
fmt='o', color='C3', lw=0.5)
ax[3].set(xlim=(0.08, 100), xticks=np.logspace(-1, 2, 4), xscale='log',
ylim=(0., 0.7), xlabel='input frequency (Hz)',
ylabel=r'$\langle r_S \rangle$')
ax[3].xaxis.set_major_formatter(ScalarFormatter())
pu.adjust_spines(ax[3], ['left', 'bottom'])
pu.adjust_ylabels(ax, x_offset=-0.2)
plt.show()