Example: example_1_COBA
Modeling neuron-glia interactions with the Brian 2 simulator Marcel Stimberg, Dan F. M. Goodman, Romain Brette, Maurizio De Pittà bioRxiv 198366; doi: https://doi.org/10.1101/198366
Figure 1: Modeling of neurons and synapses.
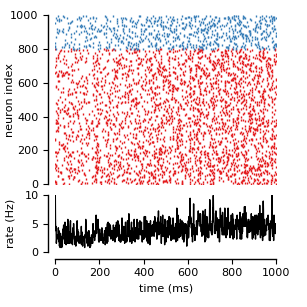
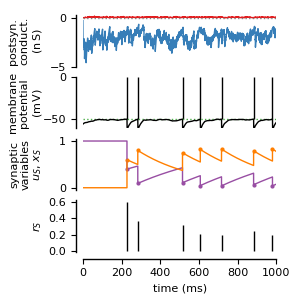
Randomly connected networks with conductance-based synapses (COBA; see Brunel, 2000). Synapses exhibit short-time plasticity (Tsodyks, 2005; Tsodyks et al., 1998).
from brian2 import *
import sympy
import plot_utils as pu
seed(11922) # to get identical figures for repeated runs
################################################################################
# Model parameters
################################################################################
### General parameters
duration = 1.0*second # Total simulation time
sim_dt = 0.1*ms # Integrator/sampling step
N_e = 3200 # Number of excitatory neurons
N_i = 800 # Number of inhibitory neurons
### Neuron parameters
E_l = -60*mV # Leak reversal potential
g_l = 9.99*nS # Leak conductance
E_e = 0*mV # Excitatory synaptic reversal potential
E_i = -80*mV # Inhibitory synaptic reversal potential
C_m = 198*pF # Membrane capacitance
tau_e = 5*ms # Excitatory synaptic time constant
tau_i = 10*ms # Inhibitory synaptic time constant
tau_r = 5*ms # Refractory period
I_ex = 150*pA # External current
V_th = -50*mV # Firing threshold
V_r = E_l # Reset potential
### Synapse parameters
w_e = 0.05*nS # Excitatory synaptic conductance
w_i = 1.0*nS # Inhibitory synaptic conductance
U_0 = 0.6 # Synaptic release probability at rest
Omega_d = 2.0/second # Synaptic depression rate
Omega_f = 3.33/second # Synaptic facilitation rate
################################################################################
# Model definition
################################################################################
# Set the integration time (in this case not strictly necessary, since we are
# using the default value)
defaultclock.dt = sim_dt
### Neurons
neuron_eqs = '''
dv/dt = (g_l*(E_l-v) + g_e*(E_e-v) + g_i*(E_i-v) +
I_ex)/C_m : volt (unless refractory)
dg_e/dt = -g_e/tau_e : siemens # post-synaptic exc. conductance
dg_i/dt = -g_i/tau_i : siemens # post-synaptic inh. conductance
'''
neurons = NeuronGroup(N_e + N_i, model=neuron_eqs,
threshold='v>V_th', reset='v=V_r',
refractory='tau_r', method='euler')
# Random initial membrane potential values and conductances
neurons.v = 'E_l + rand()*(V_th-E_l)'
neurons.g_e = 'rand()*w_e'
neurons.g_i = 'rand()*w_i'
exc_neurons = neurons[:N_e]
inh_neurons = neurons[N_e:]
### Synapses
synapses_eqs = '''
# Usage of releasable neurotransmitter per single action potential:
du_S/dt = -Omega_f * u_S : 1 (event-driven)
# Fraction of synaptic neurotransmitter resources available:
dx_S/dt = Omega_d *(1 - x_S) : 1 (event-driven)
'''
synapses_action = '''
u_S += U_0 * (1 - u_S)
r_S = u_S * x_S
x_S -= r_S
'''
exc_syn = Synapses(exc_neurons, neurons, model=synapses_eqs,
on_pre=synapses_action+'g_e_post += w_e*r_S')
inh_syn = Synapses(inh_neurons, neurons, model=synapses_eqs,
on_pre=synapses_action+'g_i_post += w_i*r_S')
exc_syn.connect(p=0.05)
inh_syn.connect(p=0.2)
# Start from "resting" condition: all synapses have fully-replenished
# neurotransmitter resources
exc_syn.x_S = 1
inh_syn.x_S = 1
# ##############################################################################
# # Monitors
# ##############################################################################
# Note that we could use a single monitor for all neurons instead, but in this
# way plotting is a bit easier in the end
exc_mon = SpikeMonitor(exc_neurons)
inh_mon = SpikeMonitor(inh_neurons)
### We record some additional data from a single excitatory neuron
ni = 50
# Record conductances and membrane potential of neuron ni
state_mon = StateMonitor(exc_neurons, ['v', 'g_e', 'g_i'], record=ni)
# We make sure to monitor synaptic variables after synapse are updated in order
# to use simple recurrence relations to reconstruct them. Record all synapses
# originating from neuron ni
synapse_mon = StateMonitor(exc_syn, ['u_S', 'x_S'],
record=exc_syn[ni, :], when='after_synapses')
# ##############################################################################
# # Simulation run
# ##############################################################################
run(duration, report='text')
################################################################################
# Analysis and plotting
################################################################################
plt.style.use('figures.mplstyle')
### Spiking activity (w/ rate)
fig1, ax = plt.subplots(nrows=2, ncols=1, sharex=False,
gridspec_kw={'height_ratios': [3, 1],
'left': 0.18, 'bottom': 0.18, 'top': 0.95,
'hspace': 0.1},
figsize=(3.07, 3.07))
ax[0].plot(exc_mon.t[exc_mon.i <= N_e//4]/ms,
exc_mon.i[exc_mon.i <= N_e//4], '|', color='C0')
ax[0].plot(inh_mon.t[inh_mon.i <= N_i//4]/ms,
inh_mon.i[inh_mon.i <= N_i//4]+N_e//4, '|', color='C1')
pu.adjust_spines(ax[0], ['left'])
ax[0].set(xlim=(0., duration/ms), ylim=(0, (N_e+N_i)//4), ylabel='neuron index')
# Generate frequencies
bin_size = 1*ms
spk_count, bin_edges = np.histogram(np.r_[exc_mon.t/ms, inh_mon.t/ms],
int(duration/ms))
rate = double(spk_count)/(N_e + N_i)/bin_size/Hz
ax[1].plot(bin_edges[:-1], rate, '-', color='k')
pu.adjust_spines(ax[1], ['left', 'bottom'])
ax[1].set(xlim=(0., duration/ms), ylim=(0, 10.),
xlabel='time (ms)', ylabel='rate (Hz)')
pu.adjust_ylabels(ax, x_offset=-0.18)
### Dynamics of a single neuron
fig2, ax = plt.subplots(4, sharex=False,
gridspec_kw={'left': 0.27, 'bottom': 0.18, 'top': 0.95,
'hspace': 0.2},
figsize=(3.07, 3.07))
### Postsynaptic conductances
ax[0].plot(state_mon.t/ms, state_mon.g_e[0]/nS, color='C0')
ax[0].plot(state_mon.t/ms, -state_mon.g_i[0]/nS, color='C1')
ax[0].plot([state_mon.t[0]/ms, state_mon.t[-1]/ms], [0, 0], color='grey',
linestyle=':')
# Adjust axis
pu.adjust_spines(ax[0], ['left'])
ax[0].set(xlim=(0., duration/ms), ylim=(-5.0, 0.25),
ylabel=f"postsyn.\nconduct.\n(${sympy.latex(nS)}$)")
### Membrane potential
ax[1].axhline(V_th/mV, color='C2', linestyle=':') # Threshold
# Artificially insert spikes
ax[1].plot(state_mon.t/ms, state_mon.v[0]/mV, color='black')
ax[1].vlines(exc_mon.t[exc_mon.i == ni]/ms, V_th/mV, 0, color='black')
pu.adjust_spines(ax[1], ['left'])
ax[1].set(xlim=(0., duration/ms), ylim=(-1+V_r/mV, 0.),
ylabel=f"membrane\npotential\n(${sympy.latex(mV)}$)")
### Synaptic variables
# Retrieves indexes of spikes in the synaptic monitor using the fact that we
# are sampling spikes and synaptic variables by the same dt
spk_index = np.in1d(synapse_mon.t, exc_mon.t[exc_mon.i == ni])
ax[2].plot(synapse_mon.t[spk_index]/ms, synapse_mon.x_S[0][spk_index], '.',
ms=4, color='C3')
ax[2].plot(synapse_mon.t[spk_index]/ms, synapse_mon.u_S[0][spk_index], '.',
ms=4, color='C4')
# Super-impose reconstructed solutions
time = synapse_mon.t # time vector
tspk = Quantity(synapse_mon.t, copy=True) # Spike times
for ts in exc_mon.t[exc_mon.i == ni]:
tspk[time >= ts] = ts
ax[2].plot(synapse_mon.t/ms, 1 + (synapse_mon.x_S[0]-1)*exp(-(time-tspk)*Omega_d),
'-', color='C3')
ax[2].plot(synapse_mon.t/ms, synapse_mon.u_S[0]*exp(-(time-tspk)*Omega_f),
'-', color='C4')
# Adjust axis
pu.adjust_spines(ax[2], ['left'])
ax[2].set(xlim=(0., duration/ms), ylim=(-0.05, 1.05),
ylabel='synaptic\nvariables\n$u_S,\,x_S$')
nspikes = np.sum(spk_index)
x_S_spike = synapse_mon.x_S[0][spk_index]
u_S_spike = synapse_mon.u_S[0][spk_index]
ax[3].vlines(synapse_mon.t[spk_index]/ms, np.zeros(nspikes),
x_S_spike*u_S_spike/(1-u_S_spike))
pu.adjust_spines(ax[3], ['left', 'bottom'])
ax[3].set(xlim=(0., duration/ms), ylim=(-0.01, 0.62),
yticks=np.arange(0, 0.62, 0.2), xlabel='time (ms)', ylabel='$r_S$')
pu.adjust_ylabels(ax, x_offset=-0.20)
plt.show()